AAFP Backs New EUAs on COVID-19 Boosters for Children
The AAFP reviewed evidence on the use of bivalent COVID-19 booster vaccines in children and adolescents, and approved action by the FDA and CDC on amended emergency use authorizations.

Vaccination

Vaccines & Diseases - Vaccinate Your Family

AAFP Gathers Latest on Boosters, Guidance, Treatment, More
Boosters For All: Keeping all children up-to-date with COVID-19 vaccine – AZ Dept. of Health Services Director's Blog

Vaccination

Immunizations

Adolescent Vaccination Archives

FDA and CDC clear updated COVID boosters for kids as young as 5 years old - CBS News

CDC OKs COVID Vaccines for Children as Young as 6 Months

FDA Authorizes COVID Vaccine Boosters For Kids 12 To 15 Years Old - CBS Miami
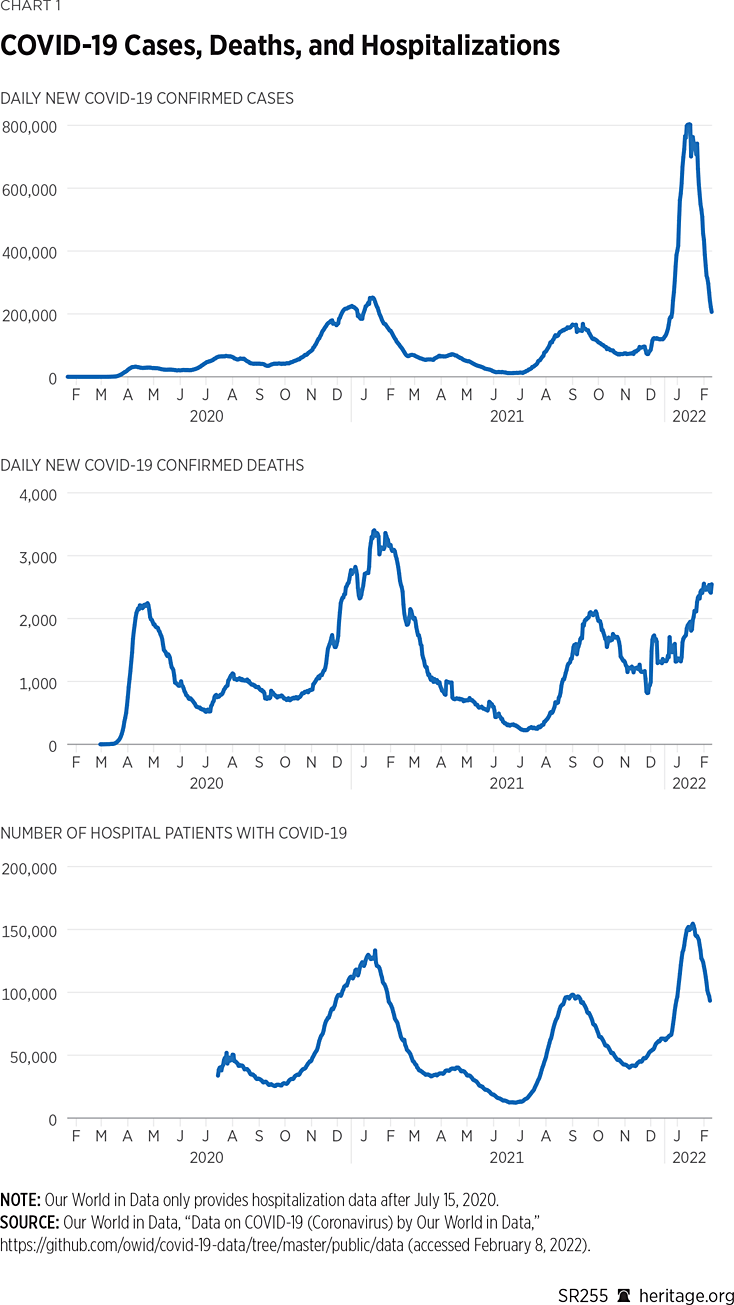
COVID-19: A Statistical Analysis of Data from Throughout the Pandemic and Recommendations for Moving On